Redox Reactions: Definition, Types, Examples, Equations, Applications, and Importance in Chemistry
In order to understand redox reactions, let us first deal with oxidation and reduction reactions individually.
Oxidation Reaction
Oxidation can be defined in two ways:
1. As the loss of electrons from a substance.
2. As the addition of oxygen or a more electronegative element, or the removal of hydrogen or a more electropositive element.
Examples of oxidation reactions include:
2S(s) + O2 (g) → SO2 (g)
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
Watch Full Lecture in Urdu / Hindi
Watch Full Lecture in English
What Is Reduction Reaction?
Reduction reactions, like oxidation reactions, can be defined in two main ways:
1. As the gain of electrons by a substance during a chemical reaction.
2. As the addition of hydrogen or a more electropositive element, or the removal of oxygen or a more electronegative element from a substance.
These definitions describe the various ways in which a substance can undergo reduction.
Below are some examples of reduction reactions:
2CH2CH2 (g) + H2 (g) → CH3CH3 (g)
2FeCl3 (aq) + H2 (g) → 2FeCl2 (aq) + 2HCl (aq)
Redox Reactions
A redox reaction can be defined as a chemical reaction in which electrons are transferred between two reactants participating in it. This transfer of electrons can be identified by observing the changes in the oxidation states of the reacting species.
An illustration detailing the electron transfer between two reactants in a redox reaction is provided below.
In the illustration below, reactant A loses an electron and is therefore oxidized, while reactant B gains that electron and is reduced.
Here’s the reaction rewritten the same way as shown in the image:
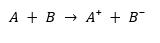
- A → undergoes oxidation (loss of electron)
- B → undergoes reduction (gain of electron)
- A⁺ = Oxidized product
- B⁻ = Reduced product
Oxidation is defined as the loss of electrons, resulting in an increase in the oxidation state of a reactant. Conversely, reduction is the gain of electrons, leading to a decrease in the oxidation state.
In redox reactions:
A species that accepts electrons and undergoes reduction is called an oxidizing agent.
A species that donates electrons and undergoes oxidation is known as a reducing agent.
Every redox reaction can be separated into two half-reactions:
1. The oxidation half-reaction, and
2. The reduction half-reaction.
When writing these half-reactions, it is essential to balance them properly, ensuring that all atoms and electrons are accurately accounted for.
Types of Redox Reactions
Decomposition Reaction
This kind of reaction involves the breakdown of a compound into different compounds. Examples of these types of reactions are
2NaH → 2Na + H2
2H2O → 2H2 + O2
All the above reactions result in the breakdown of smaller chemical compounds in the form of AB → A + B
Combination Reaction
These reactions are the opposite of decomposition reactions and hence, involve the combination of two compounds to form a single compound in the form of A + B → AB. For example,
- H2 + Cl2 → 2HCl C+O2→CO2
Displacement Reaction
In this kind of reaction, an atom or an ion in a compound is replaced by an atom or an ion of another element. It can be represented in the form of X + YZ → XZ + Y. Further displacement reactions can be categorised into
• Metal displacement reaction
• Non-metal displacement reaction
Metal Displacement
In this type of reaction, one metal displaces another metal from its compound. Such reactions are commonly used in metallurgical processes to extract pure metals from their ores.
For example, CuSO4+Zn→Cu+ZnSO4
Non-metal Displacement
In this type of reaction, hydrogen displacement is commonly observed, while oxygen displacement reactions occur rarely.
Disproportionation Reactions
Disproportionation reactions are known as reactions in which a single reactant is oxidized and reduced.
For example, P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Examples of redox reactions,
Example 1: Reaction between Hydrogen and Fluorine
In the reaction between hydrogen and fluorine, hydrogen is oxidized and fluorine is reduced. The reaction can be represented as:
H2 + F2 → 2HF
The oxidation half-reaction is: H2 → 2H+ + 2e–
The reduction half-reaction is: F2 + 2e– → 2F–
The hydrogen and fluorine ions go on to combine in order to form hydrogen fluoride.
Example 2: Reaction between Zinc and Copper
This is an example of a metal displacement reaction, where zinc displaces Cu²⁺ ions from copper sulfate solution to form copper metal, as shown in the reaction below:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The oxidation half-reaction can be written as Zn → Zn2+ + 2e–
The reduction half-reaction can be written as Cu2+ + 2e– → Cu
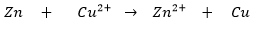
- Zn = Reducing agent
- Cu²⁺ = Oxidizing agent
- Zn²⁺ = Oxidized product
- Cu = Reduced product
Thus, zinc displaces copper from copper sulfate solution in a redox reaction, where zinc is oxidized and copper is reduced.
Download Complete Notes Below
Proudly Powered By