Nernst Equation: Definition, Derivation, Formula, Applications, Limitations, and Examples in Electrochemistry
The Nernst equation is a fundamental equation in electrochemistry that predicts the electrode potential (voltage) at which an electrochemical reaction occurs under non-standard conditions. It is used to calculate the electrode potential of a half-cell or the cell potential of a galvanic cell under non-standard conditions, based on the concentrations of the reacting species. The Nernst equation is a crucial tool for understanding the behavior of batteries, fuel cells, and other electrochemical devices, as it explains how their voltages change with varying concentrations and conditions.
Nernst Equation is given by,
E = E° – (RT/nF) × ln(Q)
Where
E is observed electrode potential
E° is the standard electrode potential
R is the gas constant
T is the temperature in Kelvin
n is the number of electrons transferred in the reaction
F is Faraday’s constant
ln(Q) is the natural logarithm of the reaction quotient
Watch full lecture in Urdu / Hindi
Watch full lecture in English
Derivation of Nernst Equation
Gibbs free energy of the cell reaction can be expressed as,
ΔG = ΔG° + RT × ln(Q)
Standard Gibbs free energy change (ΔG°) can be related to the standard electrode potential (E°) as follows,
ΔG° = -nFE°
Substituting ΔG° in the expression for ΔG,
ΔG = -nFE° + RT × ln(Q)
Solving for the observed electrode potential (E),
E = ΔG / (-nF)
E = -nFE° / nF + RT × ln(Q) / (-nF)
E = E° – (RT / nF) × ln(Q)
Calculation of Half-cell potential
For an oxidation half-cell reaction when the metal electrode gives
ion,
The Nernst equation takes the form
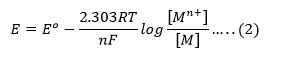
The concentration of solid metal [M] is equal to zero. Therefore, the Nernst equation can be written as
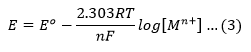
Substituting the values of R,F and T at 25℃, the quantity 2.303 RT/F comes to be 0.0591. Thus the Nernst equation (3) can be written in its simplified form as
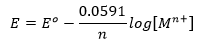
This is the equation for a half-cell in which oxidation occurs. In case it is a reduction reaction, the sign of E will have to be reversed.
Calculation of cell potential
The Nernst equation is applicable to cell potential as well. Thus,
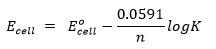
K is the equilibrium constant of the redox cell reaction.
Equilibrium Constant with Nernst Equation
By rearranging the equation, we can calculate the equilibrium constant:
Q = e((E° – E)/(RT/nF))
Applications of Nernst Equation
Electrochemistry: The Nernst equation is widely used in electrochemistry to calculate the electrode potential under non-standard conditions. It also helps in determining the concentrations of reactants and products in redox reactions based on the measured cell potential.
Biochemistry: The Nernst equation is applied in biochemistry to calculate the membrane potential resulting from the distribution of ions across biological membranes. It is especially useful for understanding the behavior of ion channels and the electrochemical gradients that drive ion transport in cells.
Analytical Chemistry: The Nernst equation is used to determine the concentration of ions in solution by relating it to the electrode potential. It is particularly useful in potentiometric methods, such as determining hydrogen ion concentration (pH) during acid-base titrations using a pH electrode.
Limitations of Nernst Equation
The Limitations of Nernst Equation are discussed below,
• The Nernst equation assumes ideal conditions like constant temperature and pressure. However, real-world systems often deviate from these conditions, affecting the accuracy of predicted cell potentials.
• The Nernst equation assumes a constant electrode potential, but in practical applications, factors like electrode polarization can cause deviations, making the actual potential differ from the theoretical value.
• The Nernst equation is best suited for reactions involving a limited number of simple ions. It may not accurately describe systems with complex species or multi-electron transfer reactions, where additional factors affect the electrode potential.
Download Complete Notes Below
Proudly Powered By