Liquid Junction Potential Explained: Definition, Calculation, Examples, and Applications in Electrochemistry
Liquid junction potential arises when two electrolytic solutions of differing concentrations come into contact. Due to this difference, ions from the more concentrated solution tend to diffuse into the less concentrated one, creating a potential difference at the junction.
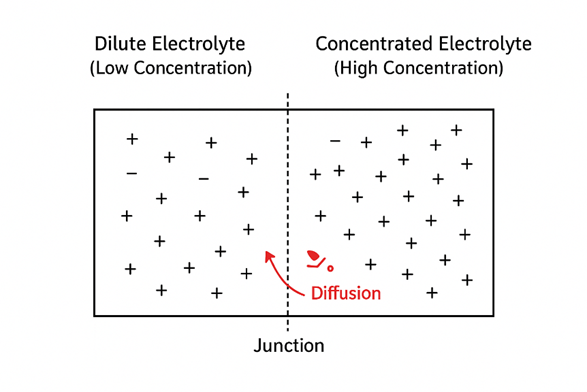
The diffusion rate of each ion is approximately proportional to its mobility in an electric field. If anions diffuse faster than cations, they move ahead into the dilute solution more quickly, leaving behind excess positive charge in the concentrated solution. As a result, the dilute solution becomes negatively charged, and the concentrated solution becomes positively charged.
This leads to the formation of an electrical double layer at the junction, with positive and negative charges accumulating on either side. As a result, a potential difference—known as the liquid junction potential or diffusion potential—develops due to the unequal movement of ions. This is a non-equilibrium potential, and its magnitude depends on the relative speeds of the migrating ions.
A salt bridge is a low-resistance device that provides electrical contact between two separate electrolyte solutions, preventing direct mixing. It typically consists of a glass U-tube filled with a gel made from potassium chloride (KCl) and agar-agar. The electrolyte used in the salt bridge must be inert—meaning it should not chemically or electrochemically react with the cell’s electrolytes. Common choices include KCl, KNO₃, NH₄NO₃, and K₂SO₄, depending on the nature of the electrolytes in the cell.
A direct liquid-liquid junction represents a thermodynamically unstable state. The unequal migration rates of cations and anions across this junction create a potential difference known as the liquid junction potential. A salt bridge helps eliminate direct contact between the two solutions, thereby minimizing this potential difference and stabilizing the electrochemical system.
Watch Full lecture in Urdu/Hindi
Watch Full lecture in English
Purpose and Function of a Salt Bridge
• A salt bridge enables ion exchange between two reservoirs without allowing the transfer of reactive species.
• Its primary functions are:
• To permit ion migration that maintains electroneutrality in both half-cells.
• To prevent the movement of reactive ions , i.e., those directly involved in the electrode reactions between the compartments.
Download Complete Notes Below
I’m very happy to read this. This is the type of manual that needs to be given and not the…
Proudly Powered By




Leave a Comment