Conductometric titrations are those in which conductance measurements are used to determine the end-point of acid-base reactions, certain displacement reactions, or precipitation reactions.
These titrations rely on the fact that the conductance of a solution at a constant temperature depends on the number of ions present and their mobility.
Principle
During a titration, one ion is replaced by another, and the difference in their ionic conductivities directly affects the overall conductivity of the solution.
It can also be observed that ionic conductance values differ between cations and anions. Ultimately, the conductivity of the solution also depends on whether a chemical reaction occurs within the electrolyte.
Watch Full lecture in Urdu/Hindi
Watch Full lecture in English
Theory
The theory behind conductometric titrations states that the end-point can be determined through conductivity measurements. In an acid-base neutralization, the initial addition of the base decreases the solution’s conductivity, as highly conductive H⁺ ions are replaced by the less conductive cations of the base.
After the equivalence point is reached, the concentration of ionic species increases, leading to a rise in the solution’s conductance. When conductance values are plotted against the volume of titrant added, two straight lines with opposite slopes are obtained. The point of intersection of these lines represents the equivalence point.
Some common examples of conductometric titrations are listed below.
- Strong acid against strong base: (HCl against NaOH)
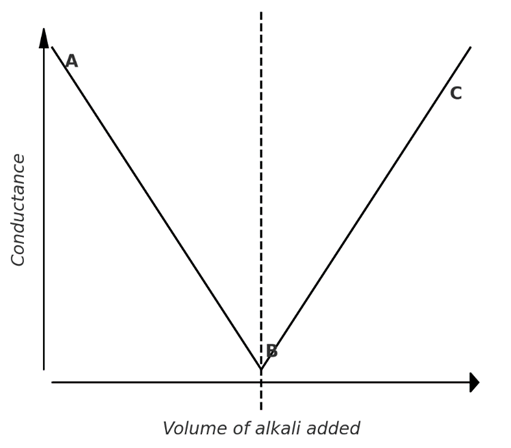
The acid (HCl) is placed in a conductivity vessel, while the alkali is taken in a burette. The electrical conductance of HCl arises from the presence of highly mobile H⁺ and Cl⁻ ions. As the alkali is gradually added, the fast-moving H⁺ ions are progressively replaced by the slower-moving Na⁺ ions, as illustrated below.
Up to the point of complete neutralization, the conductance decreases with the addition of NaOH, as the highly mobile H⁺ ions are replaced by the slower-moving Na⁺ ions. Beyond the equivalence point, any further addition of alkali introduces fast-moving OH⁻ ions, leading to an increase in conductance. When conductance is plotted against the volume of alkali added, the graph shows two straight lines intersecting at point ‘B’, which marks the end point of the titration.
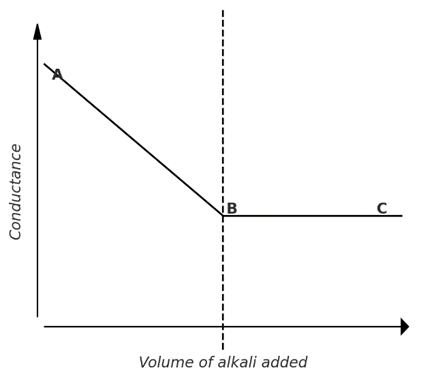
2) Weak acid against strong base: (CH3COOH against NaOH)
When a weak acid is present, its conductance is low due to limited ionization. As a strong base is added, the weakly conducting acid gradually converts into a highly ionized salt, causing a slow increase in conductance up to the equivalence point. Beyond this point, the addition of excess alkali leads to a sharp rise in conductance due to the presence of free hydroxide ions.
The graph is represented as:

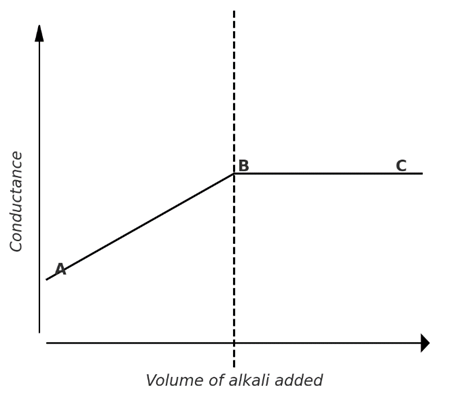
3) Strong acid against weak base: (HCl against NH4OH)
In this case, conductance initially decreases because the fast-moving H⁺ ions are replaced by the slower-moving NH₄⁺ ions. As more strong base is added, the weak acid.
Beyond end point, further addition of weakly ionized NH4OH will not cause any appreciable change in conductance. The point of intersection of curves is the end point of titration.
4) Weak acid against weak base: (CH3COOH against NH4OH)
In this titration, conductance initially increases due to the formation of ammonium acetate (CH₃COONH₄), a strong electrolyte. This gradual increase continues up to the equivalence point. Beyond the endpoint, the conductance remains nearly constant, showing no significant change.
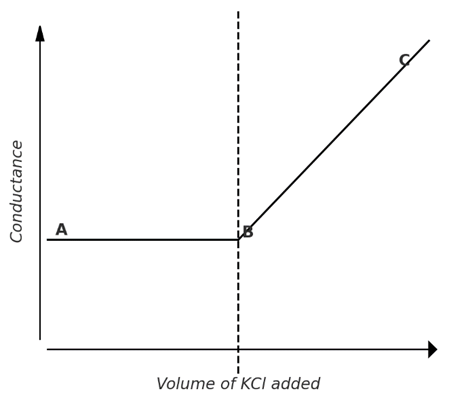
5) Precipitation titration: (AgNO3 against KCl)
The titration of silver nitrate against potassium chloride involves precipitate formation.
Since the ionic mobilities of Ag⁺ and K⁺ are nearly the same, the conductance remains almost constant up to the equivalence point. Beyond this point, the addition of KCl causes a rapid increase in conductance, as illustrated in the graph below.
Advantages of conductometric titrations:
1. Only a small quantity of solution is required for conductometric titrations.
2. Since the endpoint is determined graphically, no special precautions are needed.
3. Indicators are not required, making these titrations suitable for coloured or turbid solutions.
4. Conductometric titrations are effective for analyzing dilute solutions and weak acids.
5. They can be applied to mixtures of acids, precipitation reactions, and various other types of titrations.
6. Conductometric measurements offer higher accuracy in results.
Disadvantages of conductometric titrations:
1. High concentrations of salt in the solution can affect accuracy and lead to unreliable results.
2. The presence of other electrolytes besides the target analyte can interfere with the measurements and reduce accuracy.
3. Conductometric titrations have limited applicability in redox reactions.
Applications of conductometric titrations:
Applications of Conductometric Titration:
1. Detection of water pollution.
2. Quantitative analysis of various chemical compounds.
3. Determination of water alkalinity.
4. Measurement of water salinity.
5. Evaluation of the basicity of organic acids.
6. Estimation of deuterium ion concentration in water.
7. Determination of the solubility of sparingly soluble salts.
Download Complete Notes Below
I’m very happy to read this. This is the type of manual that needs to be given and not the…
Proudly Powered By




Leave a Comment